This audience does not have to be told that caring for a loved one with PSP can be a major burden. Two CurePSP Centers of Care have now quantified this using the Zarit Caregiver Burden Scale completed from 2014 to 2022 by 139 caregivers of 131 persons with atypical Parkinsonian disorders. The results are published in Clinical Parkinsonism & Related Disorders. Here’s the scale:
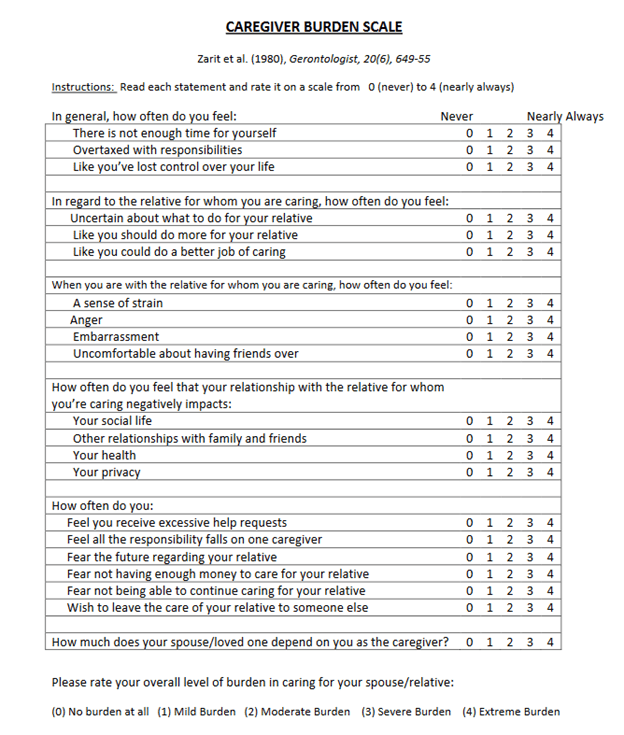
Ninety (65%) of the caregivers were women. Of the 131 patients they were caring for, 59% had PSP, 28% MSA and 13% CBS. The patients were on average about 4.5 years into their illnesses. (All three disorders have about the same average rate of progression and survival duration.) Here are the salient statistically significant results, adjusted for potential confounders as necessary:
- The average (i.e., mean) total score was 28.8 of a possible 88.
- Of the four disorders, PSP, CBS and MSA-cerebellar gave similar average scores, but scores for MSA-Parkinson were milder by a margin of about 8 points.
- Female patients regardless of diagnosis involved a greater caregiver burden than males, regardless of the caregiver gender.
- Female caregivers reported greater burden than males regardless of the patient’s gender or diagnosis.
The authors list several weaknesses of the analysis and propose a prospective study to avoid these. They include the absence of consideration of:
- the relationship between patient and caregiver
- age and any chronic illness of the caregivers
- changes in the score over time
- measures of the patient’s disability, especially falling frequency
The authors’ subjective opinion is that the patient’s cognitive and behavioral deficits are perhaps the most important determinant of the caregiver’s burden. A future study should examine this hypothesis in a formal way.
Adding these variables to the analysis would require a larger number of patient/caregiver pairs. This might be best accomplished in the context of a large treatment trial, where “secondary” and “exploratory” measures always accompany the “primary” measure of the drug’s efficacy. Alternatively, all 36 CurePSP Centers of Care could undertake such a project outside of any treatment trial, assuming adequate funding is available.
The first author of the new paper is Jessica Shurer, a medical social worker who serves as CurePSP’s Director of Clinical Affairs and Advocacy. The two senior authors are neurologists Alexander Pantelyat and Miriam Sklerov, directors of the Centers of Care at Johns Hopkins and the University of North Caroline at Chapel Hill, respectively, The three are credited with having conceptualized the study along with the second-named author, Margaret Ivancic, a medical social worker at UNC.
