Here’s great news: The new Federal spending bill, which the president has now signed into law, includes a continuation of Medicare’s payment for tele-health visits, including audio-only visits, through December 2027. It essentially cancels, for now, the planned return to Medicare’s pre-Covid telehealth policy.
This is especially important for those with PSP, where neurologists familiar with the condition are few and far between and where many of those affected have difficulty traveling, even by car.
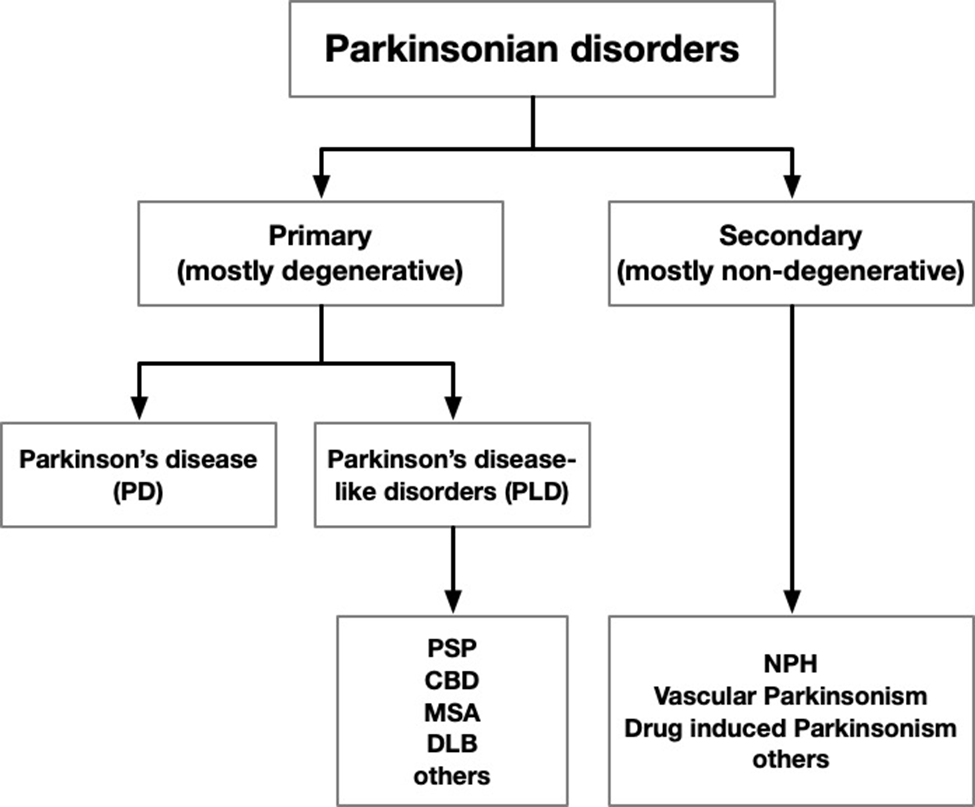
PSP is in a better position now than it was pre-Covid to adapt to telehealth. Two versions of the PSP Rating Scale that omit the items difficult to perform by video were validated in 2023. Disclosure: I was a co-author. The original version of the PSPRS, published in 2007, has 28 items and I’ll refer to it as the “PSPRS-28.” (Disclosure: I developed it and validated it with the help of statistician Pam Ohman-Strickland). One video-friendly modification, the PSPRS-25, omits the three items that require the “laying on of hands,” to use a term that shows my age. The other, the PSPRS-21, omits those plus the four others relating to eye movements and dystonia. With the sub-optimal technical situation in the patient’s home, video’s image resolution is not adequate to assess those items, even with the caregiver’s assistance.
The procedure applied the PSPRS-25 and PSPRS-21 to records from PSPRS-28 administration to two patient groups. One was the placebo group from the 12-month davunetide PSP study published in 2014 . The other was my own patients with PSP seen from 1994 to 2020, where I applied the PSPRS-28 at each visit. The result was that there was excellent correlation with the PSPRS-28 in the 12-month trial database and with long-term prognosis in the long-term database. That project was led by Drs. Alexander Pantelyat of Johns Hopkins and Anne-Marie Wills of Mass General.
Other support for video visits for PSP was provided by a study from 2020 where two neurologists independently evaluated a series of patients with various atypical Parkinsonian disorders by live video and compared diagnoses. The degree of agreement was excellent, with a kappa score (the standard statistical test for inter-rater agreement) of 0.83. A kappa of 1.0 is perfect agreement and anything over 0.75 or 0.80 is considered excellent. That study is from the University of Rochester, led by Drs. Christopher Tarolli and Jamie Adams.
It’s great to know that an accurate PSP Rating Scale exam is feasible by video, and to know that neurologists almost agree on their video diagnosis of atypical Parkinsonian disorders. But there’s a lot more to the care of someone with PSP than diagnosis and symptom rating. But hey, maybe tele-health, with a hand from AI, will learn to do all that, too. Give it a few years.