Welcome news on the PSP Platform Trial (PTP). As you recall, that’s an NIH-supported organization at about 50 US academic medical centers designed to test three anti-PSP drugs from three different drug companies simultaneously using one placebo group and one administrative apparatus. The most recent expectation is for a start in the third quarter of 2026.
Other background on the PTP:
- Here’s information from UC San Francisco, one of the headquarters sites
- Here’s CurePSP’s information
- Here’s my most recent post on the PTP
The news is that the PTP has now added the last of the three initial drugs, provisionally called LM11A-31.
It’s an oral drug that interferes with the degenerative scripts in brain cells, potentially giving them a chance to repair themselves. It accomplishes that by activating a receptor protein on the surface of brain cells called p75NTR, which modulates the cell’s response to various kinds of insults.
As you’d guess, this mechanism could apply to many other brain diseases. In fact, LM11A-31 is being investigated in animal models for Alzheimer’s, Parkinson’s and Huntington’s diseases, HIV dementia, stroke, traumatic brain injury and fetal oxygen deprivation. However, the lab animal evidence for a strong anti-tauopathy effect seems particularly strong. I’ll let the mavens explain (nerd alert):
“In AD and tauopathy mouse models, oral administration of LM11A-31 reduced excess activation of enzymes contributing to tau post-translational modifications, accumulation of multiple forms of pathological tau species and tau seeding activity, reduced elevations in multiple microglia and astrocyte markers, and decreased the loss of dendritic spines and synapses while improving performance on hippocampal-dependent memory tasks,”
The paper from which I copied/pasted that was a 6-month, Phase 2 trial of LM11A-31 in 242 people with Alzheimer’s disease. A third of that group received placebo. The drug showed acceptable safety and tolerability, with the incidence of side effects of the 200 mg dose about the same as that of the placebo and the 400 mg dose moderately higher. A secondary aim of the trial was to look for indirect evidence for slowing of progression in various neurodegeneration-related imaging and spinal fluid measures. Such evidence, promisingly, did occur more frequently in the participants on the active drug than in those on placebo.
But the Phase 2 AD trial’s potentially great news is that the rate of worsening of the cognitive loss itself as measured by each of three standard tests did seem to slow down a bit. The magnitude of that benefit did not reach statistical significance in the 6 months available, but AD trials designed to detect efficacy (as opposed to safety) generally need a year or two to have much chance of showing a statistically significant effect.
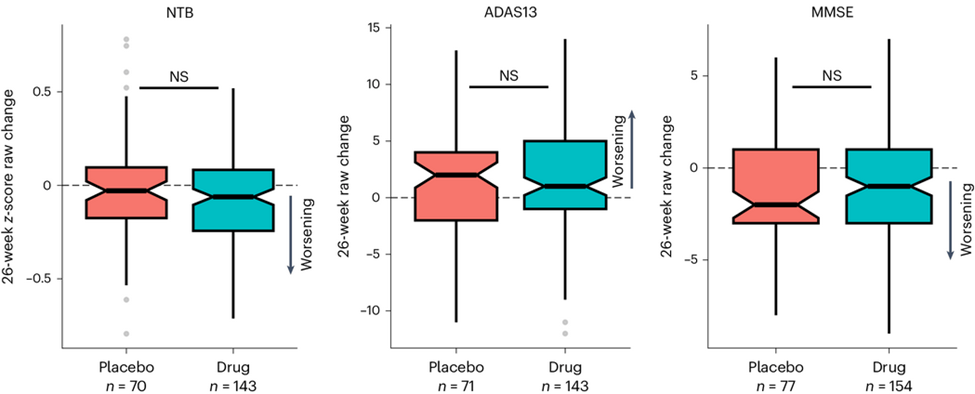
The graphs above show that trial participants with AD who received LM11A-31 progressed a bit more slowly on each of the three cognitive tests than those on placebo. However, none of the differences reached statistical significance.
NTB: Neuropsychological Test Battery; ADAS13: 13-item version of the Alzheimer’s Disease Assessment Scale; Mini-Mental Status Exam (from Shanks HRC, et al. Nature Medicine, 2024). https://www.nature.com/articles/s41591-024-02977-w
The diagram below explains the above graph’s components:
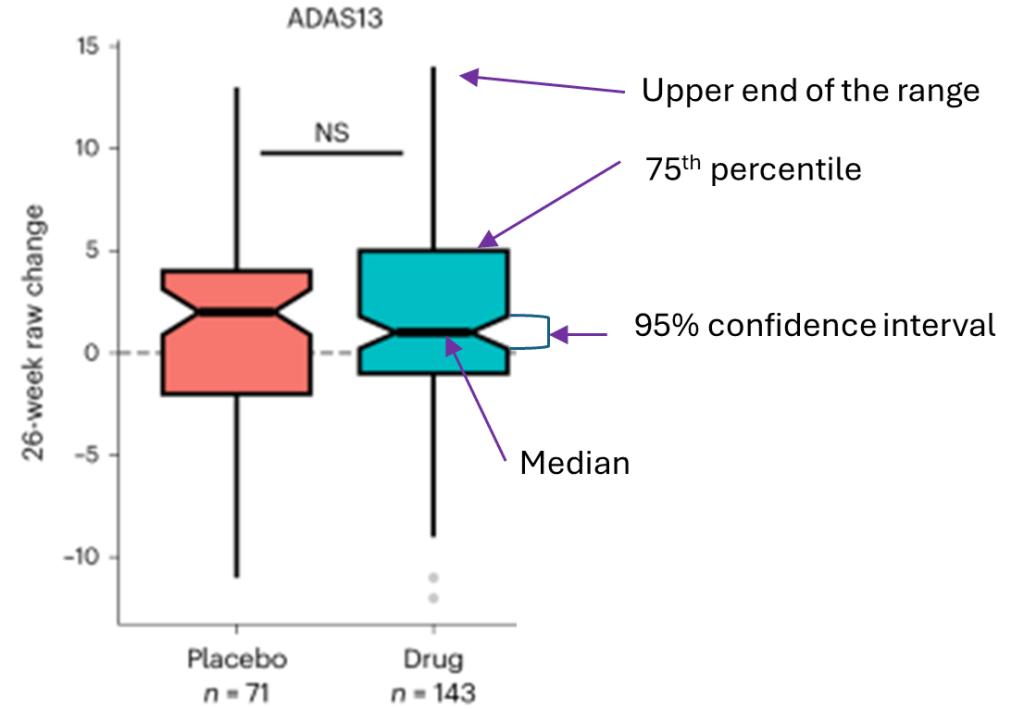
- ADAS13 is the cognitive test.
- The upward direction is worse. Downward is better.
- NS means no statistically significant difference between the active drug and placebo groups.
- The “raw” change (vertical axis label) means that the scores were not adjusted for potentially confounding factors. Such adjustment of efficacy results is routine in trials designed to assess efficacy, but not in this trial, which was designed to assess safety and tolerability.
The PSP Trial Platform’s study will also be a Phase 2 trial like the AD trial, but it will be scheduled for a full 12 months rather than six. If it starts in the third quarter of 2026 as planned, the study would be completed in mid-2029. At that point, the FDA could decide to approve the drug without a large Phase 3 trial if they feel that the results of the Phase 2 are sufficiently favorable and if there’s no other neuroprotective drug for PSP on the market at that point. The other two drugs in the PTP’s inaugural round will be AZP-2006, which enhances the breakdown of abnormal tau by the lysosomes, and AADVac1, an anti-tau active vaccine.
The sponsor of LM11A-31 is a tiny company called PharmatrophiX, Inc., founded by the scientist who discovered the drug, Frank M. Longo, MD PhD, of Stanford University. https://www.pharmatrophix.com/