In July 2023, I posted a guardedly optimistic report on the launch of a small, Phase 2a trial in South Korea of the drug GV-1001, with the generic name “tertomotide.” Three weeks ago (sorry for my delayed vigilance on your behalf), the company released some of the results. The headline was that the drug failed to show benefit in slowing the rate of progression on the PSP Rating Scale. Nevertheless, the company, GemVax, said they remained optimistic and would proceed with plans for a Phase 3 trial in North America and elsewhere.
Here’s the deal in a bit more detail. I say “a bit” because it’s not as much detail as I’d want to see. The trial was only 6 months long and the plan was for only 25 patients in each of the three groups: higher dose, lower dose and placebo. That’s too brief and too small to demonstrate a realistic degree of slowing of progression. The best longitudinal analysis of PSP to date calculated that to demonstrate a 30% slowing in a 12-month trial would require 86 patients per group. Shorter trials and more modest slowing would require even more patients than that. But early-phase trials like this are mostly about safety, not efficacy.
The results for the low-dose and placebo groups appears below, just for the PSP-Richardson patients:
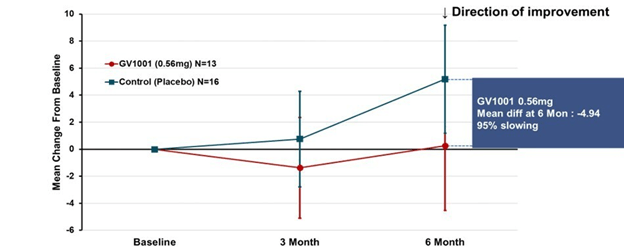
The vertical axis is the average improvement (downward) or worsening (upward) in the total PSP Rating Scale relative to the patient’s own baseline score. (On the PSPRS, 0 is the best and 100 the worst possible score, and the average patient accepted into a drug trial has a score in the mid-30s.) At 3 months, neither group showed much change. But at 6 months, the placebo group had deteriorated by 4 points but the active drug group had remained close to its baseline. So, that looks like a benefit, but the wide standard deviation (the vertical “whiskers” at 3 and 6 months) were too large to support statistical significance (i.e., to rule out the possibility of a fluke result). Hence the negative headline, but you can see why the drug company felt encouraged by the result.
A more complicated but statistically more valid way to look at the same results appears below. This graph applies to both PSP-Richardson and PSP-Parkinson patients, hence the larger Ns:
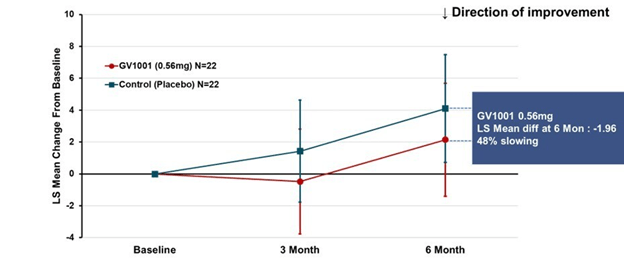
This time the vertical axis is “least square mean change from baseline.” That uses a statistical technique called “mixed-model repeated measures” to compensate for statistical noise in the results. The basic shapes of the active drug and placebo curves look similar to the raw score graph. But now, the two lines have the same slope between 3 and 6 months, suggesting that their rates of progression over that period were the same. The interval from baseline to 3 months did have different slopes, favoring active drug. So, this could mean one of 3 things:
- There’s a neuroprotective effect (i.e., a slowing of the progression rate) that lasts only 3 months, at which point the two groups proceed to progress at the same rate;
- There’s a symptomatic improvement by the 3-month point that persists to the 6-month point, but no protective effect at any point; or
- The trial’s small size, wide standard deviations, paucity of evaluations and short duration make it impossible to draw any conclusions about symptomatic or neuroprotective efficacy.
I’ll vote for Option 3.
The data for the high-dose group, which received twice the lower dose, is not presented in the company’s press release. However, the high-dose group was included in the poster at the Neuro2024 conference (CurePSP’s annual international scientific meeting) in Toronto in October. It did not show the possible benefit that the low-dose group showed. So, that’s a little discouraging, but it’s not unheard-of in pharmacology for a higher dosage regimen to do something extra via a different chemical mechanism that counteracts some of the benefit of a lower dosage. So, that doesn’t worry me much.
Now, the issue is just how safe and tolerable the drug was. The press release only says, “The safety profile of GV1001 in the Phase 2a PSP Clinical Trial was consistent with prior safety data. GV1001 was generally well-tolerated with no serious adverse events related to the drug reported.” I’ve seen the actual numbers, and the press release is right. All of the adverse events, and there were very few, were things common in this age group or complications of PSP itself.
So, that’s probably more information than you wanted about GV-1001, or maybe it’s a lot less than you’d have liked. (I’m in the latter category.) Bottom line is that the results were good enough to justify a Phase 3 trial, which is slated to start in 2025, and that’s really good news.
Note: The text in italics explaining the two graphs and detailing the drug side effects are corrections or additions to my originally posted version. I thank Roger Moon, Chief Scientific Officer of GemVax, for supplying this information after he saw the original post. These changes do not alter my conclusions.
Thank you dr. Golbe for this information. You know I’m interested in this kind of information. I’ve contacted a few companies that have given us hope with some molecules. However, when I show that I understand something about pathophysiology and pharmacology, they quickly give up on me.
Best regards,
Branko
Branko, It hardly a surprise that drug companies are uncomfortable dealing with someone of your caliber who’s not an existing staffer or consultant of their own.
Dear Dr. Golbe,
This is Woo, who previously left a comment regarding GV1001.
I deeply admire the invaluable insights you share through your blog. They have been a source of both objective perspective and hope for me as I navigate the challenges of caregiving for a loved one with Alzheimer’s disease.
The recent PSP trial results have been particularly encouraging and have given me renewed hope. I noticed on the ClinicalTrials.gov website that a study involving long-term administration for existing PSP trial participants is underway, with results expected around December this year. Additionally, the Phase 2 trial for Alzheimer’s is projected to conclude around mid-year.
As these milestones approach, I find your expert analysis and commentary to be an essential guide in interpreting these developments and maintaining an informed outlook.
I also came across a note on the CurePSP website about the platform trial testing three drugs simultaneously. If it’s not too much trouble, I would greatly appreciate your thoughts on whether GV1001 might be one of the drugs included in this trial.
Thank you for your continued dedication and the remarkable knowledge you share with the community. Your work is truly inspiring.
Warm regards,
Woo
Dear Woo: I appreciate knowing that PSP Blog has been useful, both objectively and subjectively.
As far as I know, the GV-1001 trial will not be part of the PSP Platform, but I’m not privy to GemVax’s planning, and of course the Platform’s leadership keeps their negotiations confidential until there’s a firm agreement.
Dr. G
Dr. Golbe,
I greatly appreciate this blog and all of the info you provide to the people that have dealt with PSP in their life.
I know this is off topic, but I have a question for you.
My grandfather had PSP in the early 2000s and was at a point where he was not able to walk unassisted. He certainly was not driving. Something weird happened one night – He was somehow able to get up in the middle of the night, grab car keys and drive a two miles down the road. He was found in a parking lot laying on the ground. (alive)
It was such an odd event considering he mobility; we have no idea how this could have happened considering the state he was in.
Have you seen anything like this before in PSP patients? Is there an explanation for this?
Again, thank you so much for all that you do.
Joey:
There is a phenomenon called “paradoxical kinesia” that is well known in Parkinson’s disease, though I’ve never heard of it in PSP. Nor can I find anything in the literature about it in PSP. The classic example in PD is someone chairbound in a nursing home who hears a fire alarm, gets up, runs outside, and once reaching safety, returns to their disabled state. It’s interpreted as the effect of the auditory startle reflex in opening up an alternative motor circuit bypassing the basal ganglia. Yes, it would be great to be able to put that circuit to use in a controlled way, but no researcher has really tried to do that. The unusual thing about your grandfather’s event is that his actions were more complex and prolonged than I’ve heard for paradoxical kinesia in Parkinson’s, and there was no reported sensory event to startle him (unless it was a vivid dream). But PSP differs from PD in many important ways, including having much weaker startle reflexes in general. This raises the possibility that it might not be paradoxical kinesia, but a form of sleepwalking or maybe REM behavioral disorder. People with PSP definitely can have various sleep-related motor abnormalities involving actions they can’t perform while awake.
I’ll keep alert to other people with PSP experiencing the same thing. Maybe there’s something to be discovered there. Thanks for bringing this to my attention.
Dr. Golbe
Dear Dr. Golbe,
I read Joey’s story about his Grandfather’s surprising abilities with great interest. I witnessed a similar event in which my husband performed a perhaps not quite as dramatic but, in context, still astonishing, feat.
In our case, my husband (who has been diagnosed with PSP-RS) had for months been using his rollator walker, with me assisting, to navigate anywhere in the house. It would never have occurred to either of us for him to try to walk without it. On one particular early morning, I woke up to realize that he was not in the bed, but his rollator was still beside it. I looked up to see him returning from the bathroom with no balance device whatsoever, having successfully accomplished his purpose. The trip required him to cross a relatively large bedroom and to then travel down a hallway and into a separate toilet room off of a large open bathroom.
As I say, I was astonished at the time that he was able to do this without falling. I have wondered about it many times since. The only theory I’ve had is that perhaps in his sleepy state his inhibitions and fear of falling were lowered, allowing his years-old habit of walking to the bathroom to re-emerge.
As a side note, I’ve seen other more recent examples where my husband (even while fully awake) is strikingly more clear and functional at some times than at others.
My instinct is that there may be, as you say, something to discover here.
Thank you as always for your dedication. We appreciate very much your willingness to listen.
Maura & Pedro
Thanks for that, Maura. From such anecdotes important discoveries sometimes arise. With functional MRI, perhaps the part of the brain responsible for sleep-related improvements in PSP bradykinesia can be identified. Then, knowing the neurotransmitter(s) in use by that part of the brain, perhaps drugs could be developed to enhance its action to the point of working during wakefulness.
Dr. G