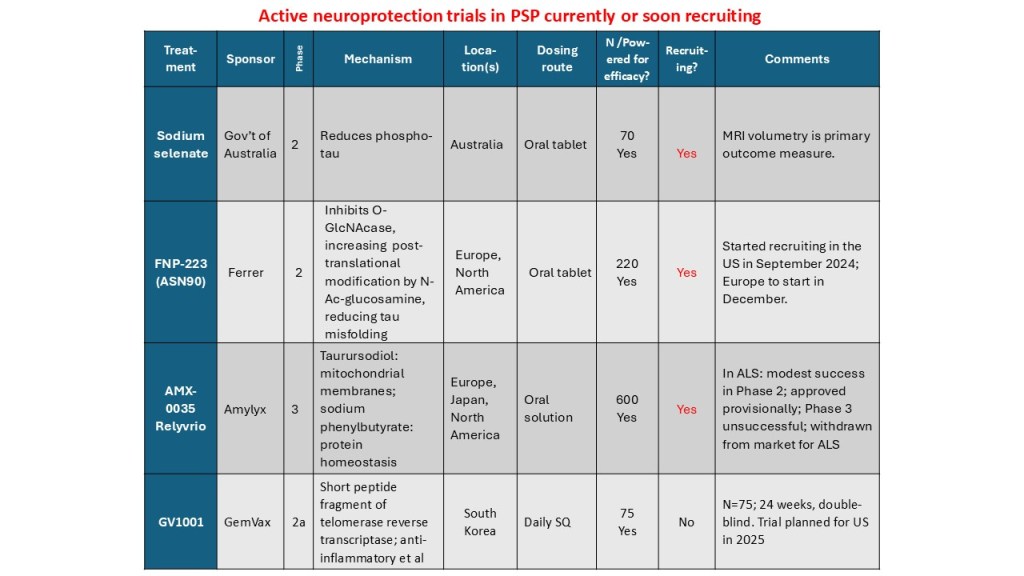
Today the Spanish drug company Ferrer issued a press release announcing the successful completion of enrollment in the PROSPER study. That’s the year-long, double-blind trial of FNP-223 that I’ve told you about in September 2025, June 2025, October 2024 and April 2024. The mechanism of action is to prevent phosphate groups from being attached to the tau protein.
Here’s Ferrer’s press release.
The recruitment required only 11 months, one month less than planned. Now, the last-enrolled patient will require 12 months to complete the trial and then the data will take a few weeks to be “cleaned.” (That sounds like scientific hanky-panky, but actually it means tracking down records for missing test results, resolving contradictory information, and getting signatures from all the neurologists on everything.) Then the statisticians take a couple of months to do their thing, producing a result. So, we’re talking early 2027.
I haven’t a clue as to whether FNP-223 is likely to work in slowing the progression of PSP. I do know that its oral administration is a plus and its mechanism of action at the subcellular level makes sense . I also know for sure that hope matters!
[Disclosure: I consulted for Ferrer in their trial design and implementation, but I have no financial stake in the trial’s outcome or the company’s success.]