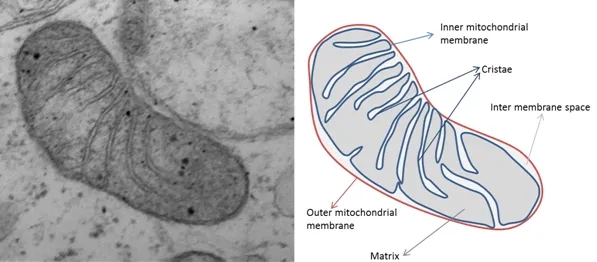
In response to a reader’s request, here’s a brief description of the mechanism of action of Relyvrio, which is a combination of two drugs, sodium phenylbutyrate and taurursodiol. The text in bold italics below is copied verbatim from the supplementary material attached to the publication reporting the results of the first ALS trial. The same explanation applies to PSP and other neurodegenerative diseases. You may feel that any treatment that claims to address all of those complex diseases is claiming too much, and you could be right. But stranger things have happened. If you want more scientific detail, see the five references below. Note that Reference 5 discusses release of cytochrome C from mitochondria. That’s a cell signalling compound that causes cells to start up their “suicide machine,” more formally called the apoptotic pathway. Cells undergo apoptosis when they’re not working well or as a normal “pruning” procedure during growth and development. Taurursodiol prevents that from happening as easily.
Endoplasmic reticulum stress or dysfunction associated with protein misfolding and aggregation has been implicated in the pathogenesis of ALS,[1] as has disruption of mitochondrial function and structure.[2] Sodium phenylbutyrate is a histone deacetylase inhibitor that has been shown to upregulate heat shock proteins and act as a small molecular chaperone, thereby ameliorating toxicity from endoplasmic reticulum stress.[3,4] Taurursodiol recovers mitochondrial bioenergetic deficits through several mechanisms, including by preventing translocation of the Bax protein into the mitochondrial membrane, thus reducing mitochondrial permeability and increasing the apoptotic threshold of the cell.[5]
1. Jaronen M, Goldsteins G, Koistinaho J. ER stress and unfolded protein response in amyotrophic lateral sclerosis—a controversial role of protein disulphide isomerase. Front Cell Neurosci 2014;8:402.
2. Mehta AR, Walters R, Waldron FM, et al. Targeting mitochondrial dysfunction in amyotrophic lateral sclerosis: A systematic review and meta-analysis. Brain Commun 2019;1:fcz009.
3. Kaur B, Bhat A, Chakraborty R, et al. Proteomic profile of 4-PBA treated human neuronal cells during ER stress. Mol Omics 2018;14:53-63.
4. Suaud L, Miller K, Panichelli AE, Randell RL, Marando CM, Rubenstein RC. 4-Phenylbutyrate stimulates Hsp70 expression through the Elp2 component of elongator and STAT-3 in cystic fibrosis epithelial cells. J Biol Chem 2011;286:45083-92.
5. Rodrigues CM, Solá S, Sharpe JC, Moura JJ, Steer CJ. Tauroursodeoxycholic acid prevents Bax- induced membrane perturbation and cytochrome C release in isolated mitochondria. Biochemistry 2003;42:3070-80.