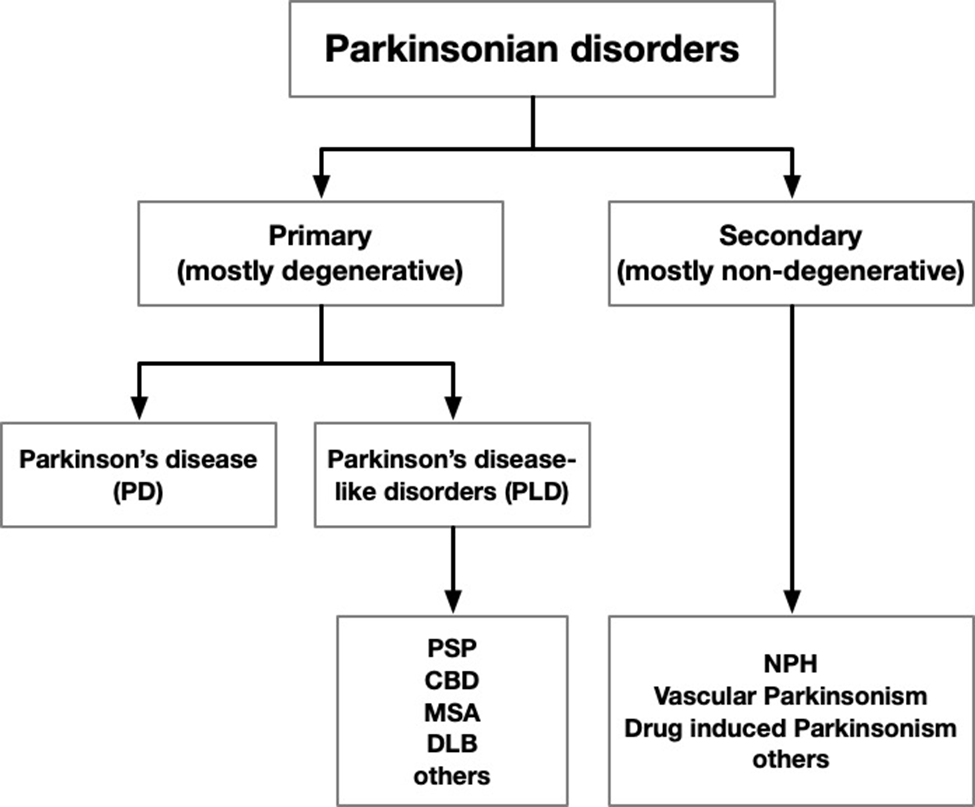
Every so often I’ll make the rounds of what’s out there on the Web by way of PSP information. So I thought a nice use of a cold day would be to share my latest observations with you all.
Every proper scientific literature review starts with a description of methods. Mine was to type “progressive supranuclear palsy” into Google and to go with the first seven sites listed. They’re allegedly the most-visited unless the sponsor has paid to be listed on top, which is the case for one of these. I added CurePSP’s main page on PSP information, which didn’t make the top seven probably because people new to PSP have heard of the other organizations but not CurePSP. I also added what ChatGPT had to say, which was a high-level summary that offered users some search terms for further details.
Clearly, CurePSP and Wikipedia were the winners, but many users could be stressed or confused by their level of detail. If that’s you, my next recommendation would be ChatGPT, but if you don’t like their very terse, outline style, I’d go with Penn Medicine. But I advise checking out more than one.
Again, like every proper scientific paper, this one has a disclaimer statement, which is that I helped write the material in the CurePSP website and am the guy lecturing in its videos mentioned here.
The sources are listed alphabetically.
| Source (alpha-betical) | Word count | Number of links to more info | Accuracy (absence of incorrect state-ments) | Compre-hensiveness (relative to others here) | Technical level (relative to others listed here) | Quality/quantity of illustrations, charts, graphs, videos | Months since last update | Com-ments |
| ChatGPT | 302 | 0 | 9 | 6 | Moderate | None | 0 | Offers examples of search terms for more detail |
| CurePSP | 4,116 | Dozens | 10 | 10 | Moderate | 2 videos of lectures, 1 of an affected family | Continual | One page in a very large website on PSP, CBS & MSA |
| Davis-Phinney Founda-tion | 1,461 | 24 | 6 | 5 | Moderate | none | 29 | Paid for Google search place-ment |
| Mayo Clinic | 1,477 | 3 | 9 | 7 | Easy | none useful | 24 | No statistics; 8th-grade reading level |
| NIH | 1,803 | 10 | 9 | 5 | Moderate | none | 10 | No statistics or lab science |
| National Health Service (UK) | 708 | 8 | 9 | 5 | Easy | none | 6 | Very super-ficial |
| Penn Medicine | 798 | 9 | 10 | 4 | Easy | none | ? | Basic but well-pre-sented |
| UC San Diego | 955 | 0 | 9 | 8 | Difficult | Includes excellent video interview with expert | ? | Outdated; few topics in text but more in video |
| Wikipedia | 4,593 | Dozens | 10 | 10 | Difficult | A useful table | Continual | Missing non-tau genetics & current diagnostic criteria |
URLs:
- ChatGPT https://chatgpt.com/c/69922393-1b50-8326-91bc-1ac1de4ecf13
- CurePSP https://www.psp.org/iwanttolearn/
- Davis-Phinney https://davisphinneyfoundation.org/blog/what-is-psp/
- Mayo Clinic https://www.mayoclinic.org/diseases-conditions/progressive-supranuclear-palsy/symptoms-causes/syc-20355659
- NIH https://www.ninds.nih.gov/health-information/disorders/progressive-supranuclear-palsy-psp
- NHS https://www.nhs.uk/conditions/progressive-supranuclear-palsy-psp/
- Penn Medicine https://www.pennmedicine.org/conditions/progressive-supranuclear-palsy
- UCSD https://neurosciences.ucsd.edu/centers-programs/movement-disorders/community/disease-overview/psp.html
- Wikipedia https://en.wikipedia.org/wiki/progressive_supranuclear_palsy